HYPOTHESIS
Loss of epidermal barrier integrity facilitates an inflammatory microenvironment promoting skin tumor formation.
Background & Significance
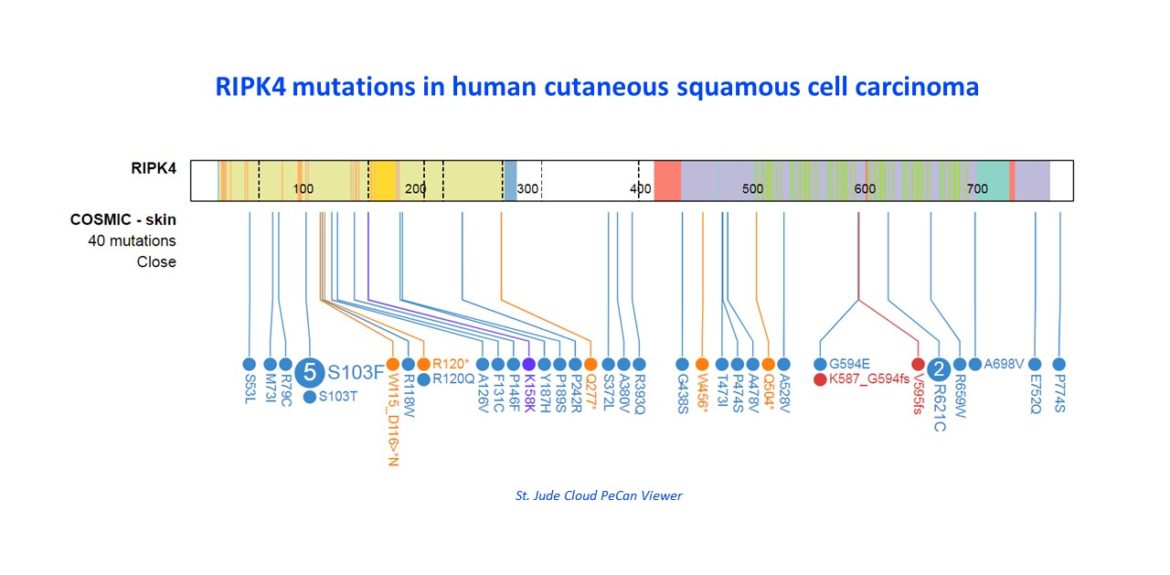
The primary cause of squamous cell carcinoma is the accumulation of ultraviolet light (UV)-induced somatic mutations. However, squamous cell cancer develops often within the context of skin infection or chronic inflammation36. In mice where the epidermal barrier is weakened by wounding, infection of flagellated bacteria drive wound-induced skin SCC development37. Also in humans there is a clear association between chronic wounds and skin cancer38. RIPK4 is a kinase involved in epidermal differentiation and homeostasis and is in the top 10 of frequently mutated genes in a set of aggressive cutaneous SCC with UV signature39. Tamoxifen-inducible deletion of RIPK4 (RIPK4iEKO) in adult epidermis causes keratinocyte-intrinsic defects in the transition from proliferation to differentiation and aberrant STAT signaling, resulting in hyperplasia, transepidermal water loss and signs of ‘subclinical’ skin inflammation. Crossing RIPK4fl/fl mice with K14-CreER; p53fl/fl mice results in SCC development within 5-8 months with 100% penetrance while K14-CreER;p53fl/fl;RIPK4+/+ mice remain tumor-free during that period, thereby defining RIPK4 as a tumor suppressor. We will investigate the contribution of commensal skin micro-organisms to mild skin inflammation and tumor development and how the inflammatory microenvironment changes during tumor development and contributes to it, comparing K14-CreER;p53fl/fl;RIPK4+/+ and K14-CreER;p53fl/fl;RIPK4-/- mice. Understanding the role of skin microbiota and skin inflammation in skin cancer is important to discover new therapeutic strategies, e.g. selective antibiotics or (genetically engineered) probiotic bacteria that compete for pathogenic tumor-promoting bacteria or inflammation-suppressing strategies. Furthermore, we will evaluate the use of inflammatory inhibitors, such as STAT3 inhibitors.
Objectives
We will characterize the inflammatory changes over time in non-tumor skin and tumor-bearing skin in the K14-CreER;p53fl/fl;RIPK4fl/fl model;
Complementary, we will study the changes in the transcriptome of epidermal keratinocytes from non-tumor skin and tumors to identify the deregulated inflammatory pathways;
Preliminary data suggest epidermal infiltration of CD8+cells. We will test whether these cells could be a source of IL-17 production thereby promoting the inflammatory status of the skin, potentially contributing to skin cancer40;
We will functionally study the contribution of the cutaneous microbiome to immune activation and tumor promotion, by housing the mice in a germ-free environment;
We will test the potential of STAT3 inhibitors to reduce tumor growth in K14-CreER;p53fl/fl;RIPK4fl/fl mice..
Enrolment in Doctoral degree(s):
- Ghent University (awards doctoral degrees to VIB students)
